| Korean J Hepatol > Volume 18(1); 2012 > Article |
ABSTRACT
Pegylated interferon and ribavirin combination therapy is accepted as the standard antiviral treatment for chronic hepatitis C regardless of HCV genotype. This combination therapy achieves higher response rates than previous therapy, but, nevertheless, a large proportion of patients suffer from treatment failure or adverse events. Recent clinical studies of viral kinetics during antiviral treatment have led to the introduction of response-guided therapy, the concept of 'customized therapy depending on viral response', which focuses on modulation of the treatment period depending on the viral response to create a sustained viral response without unnecessary medication and costs. New upcoming direct-acting antivirals (DAAs) maximize response rate, and triple therapy including DAAs along with pegylated interferon and ribavirin combination therapy could soon be the standard therapy. In this article, we reviewed the factors affecting treatment, response guided treatment, retreatment after failure of standard treatment, management of adverse events during treatment, and new treatment options.
Chronic hepatitis C affects an estimated 1-2% of the Korean population and is one of the leading causes of liver cirrhosis and hepatocellular carcinoma in Korea.1 Over the past decade, pegylated interferon (peginterferon) and ribavirin combination therapy has become the standard antiviral treatment for chronic hepatitis C regardless of hepatitis C virus (HCV) genotype. Although standard peginterferon and ribavirin combination therapy achieves better response rates than previous interferon and ribavirin combination therapy or peginterferon monotherapy, a high proportion of patients still suffer from treatment failure or adverse effects of the therapy.
The recent results of clinical trials show that there has been much progress in the treatment of chronic hepatitis C. Viral kinetics during antiviral treatment has emerged as an important predictor of treatment response and is used to guide treatment: the more rapidly HCV RNA disappears during treatment, the higher the response rate to treatment is. This suggested that the viral response to treatment could be used to modify treatment duration. Response-guided therapy, the concept of 'customized therapy depending on viral response', which modifies the treatment period depending on the viral response, is a newly emerging 'proof of concept'. Shorter treatments would not only improve overall tolerability, but also reduce unnecessary medication and expense.
In this article, we reviewed recently updated American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) practice guidelines for chronic hepatitis C,2-4 and compared them with Korean data. Upcoming DAAs were also mentioned. We focused on the factors affecting treatment, response guided treatment, retreatment after failure of standard treatment, management of adverse events during treatment, and new treatment options.
With the development of peginterferon, treatment outcomes have improved and combination therapy with peginterferon and ribavirin is currently acknowledged to be the standard antiviral treatment of chronic hepatitis C.
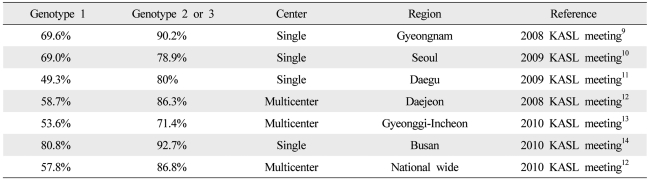
The viral genotype is one of the most important factors affecting the duration and outcome of antiviral treatment.2 Among the six genotypes of HCV, 48 week combination therapy is recommended as standard treatment for genotypes 1 and 4, whereas 24 week combination therapy is recommended for genotypes 2 and 3.1 Further studies are needed to define the standard duration for genotype 6, the most common genotype in Asia. In Western countries, the reported sustained virological response (SVR) rate is 40-50% for genotype 1 and 70-80% for genotypes 2 and 3.5 A higher response rate is reported in Korea: the SVR rate of genotype 1 is around 60-70% and those of genotypes 2 and 3 reach 80-90% (Table 1). However more prospective studies are needed in Korea because most of the studies have been retrospective and there are many differences in treatment outcomes by research institutes.
A low baseline HCV RNA level before treatment as well as the viral genotype is important factors affecting treatment outcome, and has considerable influence on decisions about duration of treatment in patients showing rapid virological responses. A low HCV RNA level is defined as <600,000 IU/mL by AASLD and 400,000-800,000 IU/mL by EASL.4
A host genetic polymorphism upstream of the IL28B gene on chromosome 19 has been recently identified as a strong indicator of a SVR to combination therapy with peginterferon alpha and ribavirin and spontaneous clearance of acute HCV infection, particularly in asymptomatic patients.6,7 The IL-28B polymorphism can help explain individual and racial differences in responses to standard combination therapy. However, additional studies are needed because IL-28B as a predictor has some disadvantages such as low accuracy in predicting treatment success in individual patients.
Other factors affecting the outcome of treatment include the stage of fibrosis, body mass index, insulin resistance, age, gender, and co-infection with another hepatotropic virus or with HIV.8
Undetectable HCV RNA in a sensitive assay at treatment week 4 is referred to as rapid virological response (RVR).4 A sensitive HCV RNA assay is defined as one with a lower limit of detection of 50 IU/mL.4 It is known that the probability of a SVR is high regardless of viral genotype when RVR is attained. Although early termination of antiviral therapy may be considered in some selected patients with RVR, absence of RVR does not justify extending the duration of treatment because of its low predictability.15-18
An early virological response (EVR) is defined as a 2 log reduction of HCV RNA levels or the disappearance of HCV RNA at treatment week 12. A partial EVR (pEVR) is defined as detectable but at least 2 log decrease in HCV RNA levels compared to baseline level, and a complete EVR (cEVR) is defined as undetectable HCV RNA at treatment week 12.2 A virological response at treatment week 12 is known as a predictor of SVR in genotype 1 chronic hepatitis C.19,20
The AASLD practice guidelines recommend that treatment should be stopped if HCV RNA is detectable at treatment week 24 and extended to 72 weeks if HCV RNA is negative at treatment week 24 in genotype 1 patients with pEVR. However, the EASL practice guidelines recommend that the HCV RNA level should be measured at treatment week 4 and 12 regardless of genotype and treatment be stopped in genotypes 2 and 3 as well as 1 if there is not at least a 2 log decrease of HCV RNA from baseline.4
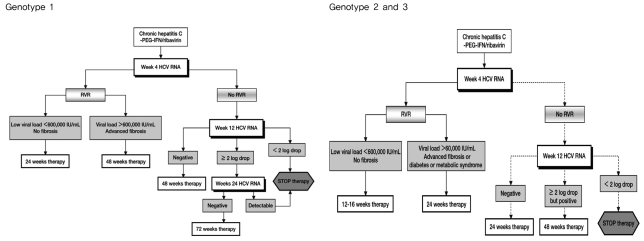
In genotype 1 chronic hepatitis C, it is reported that the treatment outcomes of 24 week short term therapy are comparable to those of 48 week standard therapy in patients with RVR and low pre-treatment HCV RNA levels (<600,000 IU/mL).16,21-23 There have been two studies of short term therapy in genotype 1 patients in Korea. One study of 50 patients with RVR reported that the number of SVR with 24 week short term therapy did not differ from that with 48 week therapy.24 The other retrospective study of 343 patients reported that all patients with low HCV RNA levels before treatment and RVR after treatment attained SVR with 24 week short term therapy.14 The recent EASL practice guidelines recommend 24 week therapy for patients who have low pre-treatment HCV RNA levels (400,000-800,000 IU/mL) and attain RVR.4 Short term therapy can be considered selectively in genotype 1 patients with low baseline HCV RNA levels who achieve RVR when maintaining the therapy is difficult because of adverse effects (Fig. 1). However, additional prospective randomized control studies are needed in Korea.
In recent studies with genotype 2 and 3 chronic hepatitis C, shortening of treatment from 24 to 16 weeks resulted in the same treatment outcomes in terms of SVR in patients who attained RVR.2,17,18 In addition, a retrospective Korean study of 163 chronic hepatitis C patients found no difference in SVR between 16 week short term therapy and 24 week standard therapy (96.8% vs. 95.1%) in patients with genotype 2 or 3 who had reached RVR.14 EASL practice guidelines recommend short term therapy of 12-16 weeks be considered in patients with RVR and low baseline viral loads (<400,000-800,000 IU/mL), but the evidence for the equal efficacy of shortened treatment is insufficient in patients with negative predictors of response such as advanced fibrosis and cirrhosis, metabolic syndrome, insulin resistance, and hepatic steatosis.4 In summary, short term therapy can be considered selectively in genotype 2 and 3 patients if they have RVR, low baseline viral loads, and no negative response predictors (Fig. 1).
A delayed viral response (DVR) is defined as undetectable HCV RNA at treatment week 24 in genotype 1 patients with pEVR. In recent studies, 72 week extended therapy yielded significantly more SVR than 48 week standard therapy in genotype 1 patients with DVR.25,26 In Europe, 72 week extended therapy is recommended for genotype 1 patients showing DVR (Fig. 1).4 Another study obtained the opposite result27 and there are no data in Korea yet. Therefore, extended therapy for genotype 1 patients should be used with care, taking into account the patients' characteristics before treatment, adverse effects of treatment, compliance and socio-economic circumstances of the patients as well as virological responses to treatment.
EASL practice guidelines recommend 48 week extended therapy for genotype 2 and 3 patients with pEVR (Fig. 1).4 However, study results for extended therapy for genotypes 2 and 3 are very limited and domestic data are insufficient. For genotype 4 patients, therapy as for genotype 1 is recommended, but there are no recommendations for 'response guide treatment' relating to genotypes 5 and 6.
A quarter of patients who receive standard therapy with peginterferon and ribavirin experience treatment failure and do not attain SVR. Treatment failure can be divided into non-response and relapse. Studies of retreatment with peginterferon and ribavirin have reported that SVR rate was 42% in the relapsed patients who had treated with non-pegylated interferon and ribavirin,28 and 33% in the relapsed patients who had treated with peginterferon and ribavirin.29 There have been two large retreatment clinical trials on non-responders after 48 week standard treatment (EPIC-3, REPEAT). The SVR rates after retreatment was as low as 6% in the EPIC-3 program and 9% in the REPEAT trial. However, the SVR rates did increase significantly but only to 16% when 72 week extended treatment was performed in the REPEAT trial.30 Interestingly, treatment success rates were relatively high in both the EPIC-3 and REPEAT trials when EVR was obtained after retreatment.
Two retrospective studies regarding the retreatment of Korean patients have been published as abstracts. The results in summary are as follows. Among patients who had used peginterferon and ribavirin, SVR rate was 0% (n=1) in the non-responders and 70% (n=10) in the relapsed patients. Among patients who had used non-pegylated interferon and ribavirin, SVR rate was 17.6% (n=17) in non-responders and 81.3% (n=16) in relapsed patients.31,32
In conclusion, retreatment with peginterferon and ribavirin should be considered in patients who received non-pegylated interferon or peginterferon monotherapy.2 However, when SVR is not attained despite an adequate period of treatment with peginterferon and ribavirin, careful consideration should precede retreatment with the same drugs. In Europe, retreatment is not recommended for non-responders to standard therapy that includes peginterferon. In such cases, triple combination therapy including a protease inhibitor which is expected to be released onto the market soon may be considered.
Adverse effects associated with combination therapy with interferon and ribavirin are relatively frequent. It has been reported that 10-14% of patients discontinued treatment because of adverse effects.20,33
A decrease in hemoglobin develops within the first 4 weeks after initiation of the therapy and larger doses of ribavirin result in more severe decrease. Ribavirin is contraindicated in the patients with chronic renal failure because the risk of hemolytic anemia is high by the accumulation of ribavirin. It is currently recommended to reduce the ribavirin dose to 600 mg if Hb decreases to <10 g/dL and to discontinue ribavirin if Hb is <8.5 g/dL. However, as SVR is higher in patients treated with high dose ribavirin in the initial phase and decreases dramatically when the dose is reduced within the first 12 weeks of treatment, careful consideration is needed before making a decision to reduce the ribavirin dose in asymptomatic anemic patients. Although with a lack of evidence about whether the use of erythropoietin (EPO) increases treatment success, the use of EPO in the initial phase of treatment (within the first 12 weeks) may decrease the frequency of ribavirin dose reduction, and improve the general condition of patients, and thus increase the drug compliance and the SVR. Therefore, selective use of EPO should be considered if hemoglobin is <10 g/dL.
Neutropenia is defined as <1,500 neutrophils/mm3 (ANC) and severe neutropenia is defined as ANC <500/mm3 in peripheral blood. Both occur in 18-20% and 4% of the patients during treatment respectively. It was recommended in a large study to halve the dose of peginterferon if ANC is reduced to <750/mm3 and to discontinue peginterferon if ANC is reduced to <500/mm3. However, no accurate clinical guidance regarding neutropenia has been established.20,33,34 Granulocyte colony stimulating factor (G-CSF) is often used in severe neutropenic patients with fever after anti-cancer therapy, and has been shown to reduce mortality associated with infections. While a number of clinicians have tried using G-CSF in the antiviral therapy of chronic hepatitis C, cases other than hepatic cirrhosis patients requiring G-CSF are rare as severe infections are not common during the therapy even in cases of neutropenia.35 In the studies that used 150-300 µg of G-CSF weekly, increase in ANC, not in SVR was demonstrated.36,37
As for platelets, it is recommended to halve the dose of peginterferon if they decline to <50,000/mm3 and to discontinue peginterferon if they are <30,000/mm3. In a phase II clinical trial, eltrombopag, an oral thrombopoietin mimetic, was shown to increase platelet count in type C hepatic cirrhosis patients and significantly increased the number of patients who were able to complete 12 week antiviral treatment.38 A phase III clinical trial is required to evaluate its effects on SVR.
The most studied DAAs against hepatitis C virus are telaprevir (TVR) and boceprevir (BOC), which are NS3/4 protease inhibitor.
Although TVR induced rapid and extensive virus inhibition in 14 day monotherapy in phase I clinical trial (a 4.4 log reduction from that before treatment) and awakened great expectations, virological breakthrough due to the emergence of drug-resistant strains occurred in a number of patients who received it as monotherapy. Therefore trials to improve SVR by combining TVR with peginterferon and ribavirin have been undertaken. In the PROVE1 and PROVE2 trials, a marked increase of SVR was observed when this combination was used for the initial 12 weeks in treatment-naive genotype 1 patients (60-69% vs. 48%), and in the PROVE3 study, a notable increase of SVR was seen when the same treatment was used in treatment-experienced genotype 1 patients (51-53% vs. 14%).39-41
BOC, a reversible covalent inhibitor of the NS3/4 protease similar to telaprevir, had a strong antiviral effect in vitro, and was effective when combined with peginterferon. In patients with genotype 1 hepatitis C who had not responded to the initial therapies in phase I clinical trial, the combination of BOC with peginterferon decreased HCV RNA more effectively than monotherapy with BOC or peginterferon.42,43 Recently, the results of the comparison of triple therapy (BOC plus standard therapy) with standard therapy in 1097 patients with genotype 1 chronic hepatitis C have been reported (SPLINT-2 study).42 In that trial, SVR rate for the 24 week and 44 week treatment groups receiving the boceprevir-containing regimen were 66% and 67%, respectively, which were significantly higher than the 40% in the standard therapy. The addition of BOC to peginterferon-ribavirin also resulted in significantly higher SVR rate than that of peginterferon-ribavirin alone in previously treated genotype 1 chronic hepatitis C patients (66% vs. 21%, respectively).
The use of BOC or TVR in combination with peginterferon alfa and ribavirin is accepted as the optimal therapy for genotype 1 in recently updated AASLD guideline.3 Triple therapy involving the addition of a protease inhibitor or other DAAs to the present standard therapy could soon become the standard treatment. In particular, a role for protease inhibitors may be anticipated in the retreatment of patients in whom peginterferon and ribavirin combination therapy have failed.
The goal of antiviral treatment in chronic hepatitis C is eradication of HCV to prevent progression of the liver disease. Antiviral therapy based on peginterferon and ribavirin combination therapy achieves an acceptable response rate, and response guided therapy improves response rate and tolerability by reducing medication and expense. With the improvement of response rate and tolerability of this treatment chronic hepatitis C with normal liver function has become widely accepted as the treatment indication. The recently introduced DAAs have raised response rates, and triple therapy with a DAA added to peginterferon and ribavirin combination therapy is already accepted as the optimal therapy for genotype 1 in AASLD guideline.3 It could soon become the standard therapy.
Acknowledgements
The authors thank Professor Dong Jin Suh, Prof. Kwang Hyub Han, and all National Conquer C Coalition members for their support and helpful comments.
Abbreviations
AASLD
American Association for the Study of Liver Disease
ANC
Absolute neutrophil count
BOC
boceprevir
cEVR
complete EVR
DAAs
direct-acting antivirals
DVR
delayed viral response
EASL
European Association for the Study of the Liver
EPO
erythropoietin
EVR
early virological response
G-CSF
granulocyte colony stimulating factor
HCV
hepatitis C virus
Peginterferon
pegylated interferon
pEVR
partial EVR
RVR
rapid virological response
SVR
sustained virological response rate
TVR
telaprevir
REFERENCES
1. Shin HR, Hwang SY, Nam CM. The prevalence of hepatitis C virus infection in Korea: pooled analysis. J Korean Med Sci 2005;20:985-988. 16361809.



2. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374. 19330875.



3. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. American Association for Study of Liver Diseases. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:1433-1444. 21898493.



4. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245-264. 21371579.


5. Afdhal NH, McHutchison JG, Zeuzem S, Mangia A, Pawlotsky JM, Murray JS, et al. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology 2011;53:336-345. 21254181.


6. Ruiz-Extremera A, Muñoz-Gámez JA, Salmerón-Ruiz MA, de Rueda PM, Quiles-Pérez R, Gila-Medina A, et al. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology 2011;53:1830-1838. 21413051.


7. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105-1109. 19749757.


8. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350-1359. 16905701.



9. Kim KT, Han SY, Kim JH, Yoon HA, Baek YH, Kim MJ, et al. Clinical outcome of pegylated interferon and ribavirin therapy for chronic hepatitis C. Korean J Hepatol 2008;14:36-45. 18367856.


10. Oh IS, Lee HW, Kim HJ, Baek EK, Kim KS, Lee SY, et al. Effect of pegylated interferon alpha-2a and ribavirin combination therapy in chronic hepatitis C. Korean J Hepatol 2009;15(Suppl 3):S215 [Abstract].

11. Lee DS, Park SY, Lee HS, Choi SY, Jung DW, Shon HS, et al. Outcome of combination therapy of pegylated inerferone with ribavirin in chronic hepatitis C: single center study. Korean J Hepatol 2009;15(Suppl 3):S134 [Abstract].
12. Kim JI, Kim SH, Lee BS, Lee HY, Lee TH, Kang YW, et al. Efficacy of initial treatment with peginterferon alpha-2a versus peginterferon alpha-2b in combination with ribavirin in naive chronic hepatitis C patients living in Daejeon and Chungcheong Province in Korea: a comparative study. Korean J Hepatol 2008;14:493-502. 19119244.


13. Park SH, Park CK, Lee JW, Kim YS, Jeong SH, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea; a multi-center, retrospective observational study. Korean J Hepatol 2010;16(Suppl 3):S58 [Abstract].



14. Song YJ, Lee YJ, Choi BJ, Choi SB, Kim JH, Jung EU, et al. Tailored therapy for treatment-naive chronic hepatitis C with pegylated Interferon-α and ribavirin : real practice experience. Korean J Hepatol 2010;16(Suppl 3):S57 [Abstract].

15. Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Gonçales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol 2005;43:425-433. 15990196.


16. Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology 2006;43:954-960. 16628671.


17. Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol 2011;55:69-75. 21145856.


18. Sarrazin C, Shiffman ML, Hadziyannis SJ, Lin A, Colucci G, Ishida H, et al. Definition of rapid virologic response with a highly sensitive real-time PCR-based HCV RNA assay in peginterferon alfa-2a plus ribavirin response-guided therapy. J Hepatol 2010;52:832-838. 20385421.


19. Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003;38:645-652. 12939591.


20. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982. 12324553.


21. Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: A randomized controlled trial. Hepatology 2008;47:43-50. 18069698.


22. Yu ML, Dai CY, Huang JF, Chuang WL. Rapid virological response to peginterferon and ribavirin for hepatitis C genotype 1: the role of weight-based ribavirin exposure. Hepatology 2008;48:1019-1020 author reply 1020-1021. 18756469.


23. Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, et al. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol 2006;44:97-103. 16290907.


24. Kim MN, Park JY, Kim DY, Ahn SH, Chon CY, Han KH. 24-week therapy with peginterferon plus ribavirin in rapid virologic responders for genotype 1 chronic hepatitis C. Korean J Hepatol 2010;16(Suppl 3):S56 [Abstract].
25. Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology 2007;46:1688-1694. 18046717.


26. Sánchez-Tapias JM, Diago M, Escartín P, Enríquez J, Romero-Gómez M, Bárcena R, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 2006;131:451-460. 16890599.


27. Buti M, Lurie Y, Zakharova NG, Blokhina NP, Horban A, Teuber G, et al. Randomized trial of peginterferon alfa-2b and ribavirin for 48 or 72 weeks in patients with hepatitis C virus genotype 1 and slow virologic response. Hepatology 2010;52:1201-1207. 20683847.


28. Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC Jr, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol 2005;100:2453-2462. 16279900.


29. Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology 2009;136:1618-1628. 19208349.


30. Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandão-Mello CE, et al. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med 2009;150:528-540. 19380853.


31. Kang P, Paik SW, Shin DH, Gil JS, Song SM, Gwak GY, et al. Pegylated interferon plus ribavirin retreatment in Korean chronic hepatitis C patients. Korean J Hepatol 2007;13(Suppl 3):S41 [Abstract].
32. Moon SS, Kang HG, Seo JA, Jung EU, Lee SH, Park SJ, et al. 24 weeks treatment with pegylated interferon alfa plus ribavirin may be possible in genotype 1 chronic hepatitis C patients with rapid virological response who have low pretreatment viremia. Korean J Gastroenterol 2010;56:33-38. 20664316.


33. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965. 11583749.


34. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355. 14996676.


35. Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology 2002;36:1273-1279. 12395340.


36. Koirala J, Gandotra SD, Rao S, Sangwan G, Mushtaq A, Htwe TH, et al. Granulocyte colony-stimulating factor dosing in pegylated interferon alpha-induced neutropenia and its impact on outcome of anti-HCV therapy. J Viral Hepat 2007;14:782-787. 17927614.


37. Younossi ZM, Nader FH, Bai C, Sjogren R, Ong JP, Collantes R, et al. A phase II dose finding study of darbepoetin alpha and filgrastim for the management of anaemia and neutropenia in chronic hepatitis C treatment. J Viral Hepat 2008;15:370-378. 18194172.


38. McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med 2007;357:2227-2236. 18046027.


39. Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009;360:1839-1850. 19403903.


40. McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009;360:1827-1838. 19403902.


41. McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med 2010;362:1292-1303. 20375406.


Figure 1
Response-guided therapy in chronic hepatitis C. There is weak evidence that the algorithm for genotype 1 can also be applied to genotype 4, and there is also weak evidence that the algorithm for genotypes 2 and 3 can be applied to genotypes 5 and 6, excluding 12-16 week therapy. The dotted lines indicate weak evidence. PEG, pegylated; IFN, interferon; HCV, hepatitis C virus; RVR, rapid virological response.
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




