1. Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164-176. 8550036.


3. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. 1244564.


4. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-470. 10075613.


5. Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis 2003;41:269-278. 12552488.


6. Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med 1994;154:201-205. 8285815.


7. Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005;11:336-343. 15719386.


8. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534-2543. 14514731.


9. Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 2005;16:3046-3052. 16148039.


10. Lopez-Hoyos M, San Segundo D, Benito MJ, Fernandez-Fresnedo G, Ruiz JC, Rodrigo E, et al. Association between serum soluble CD30 and serum creatinine before and after renal transplantation. Transplant Proc 2008;40:2903-2905. 19010142.


11. Bonventre JV. Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol 2007;156:213-219. 17464130.


12. Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta 2002;323:121-128. 12135813.


13. Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int 2004;66:1115-1122. 15327406.


17. Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 2003;18:2024-2031. 13679476.


18. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 2004;64:25-30. 15025426.


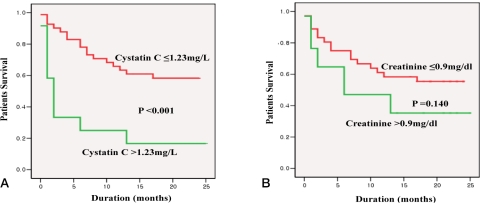
19. Seo YS, Jung ES, An H, Kim JH, Jung YK, Yim HJ, et al. Serum cystatin C level is a good prognostic marker in patients with cirrhotic ascites and normal serum creatinine levels. Liver Int 2009;29:1521-1527. 19725889.


20. Tian S, Kusano E, Ohara T, Tabei K, Itoh Y, Kawai T, et al. Cystatin C measurement and its practical use in patients with various renal diseases. Clin Nephrol 1997;48:104-108. 9285147.

21. Hellerstein S, Berenbom M, Erwin P, Wilson N, DiMaggio S. The ratio of urinary cystatin C to urinary creatinine for detecting decreased GFR. Pediatr Nephrol 2004;19:521-525. 15015062.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




