| Korean J Hepatol > Volume 17(1); 2011 > Article |
ABSTRACT
Background/Aims
E-cadherin is involved in intercellular binding and cellular polarity formation. Snail is a key regulator of the epithelial-mesenchymal transition and is closely associated with tumor invasiveness due to its ability to suppress E-cadherin expression. We investigated the expressions of E-cadherin and Snail in hepatocellular carcinoma (HCC) tissue to determine the clinical significance of these proteins in HCC.
Methods
Immunohistochemistry was used to examine the expressions of E-cadherin and Snail in resected tissues from 59 patients diagnosed with HCC. We also evaluated the relationship between the expressions of these two molecules in HCC tissue and clinicopathologic factors in the patients.
Results
Immunohistochemistry showed that Snail was stained in 20.3% of the HCC tissues and 3.4% of noncancerous tissues. Snail was not stained in the area of E-cadherin expression. The expression of Snail in the HCC tissue was associated with poorly differentiated HCC (P=0.028). The expression of Snail without E-cadherin staining in HCC tissue was significantly associated with postoperative HCC recurrence (P=0.013).
Hepatocellular carcinoma (HCC) has been known to be the 3rd frequent cancer in Korea, and the mortality due to HCC has been reported to be high.1 When detected in the advance stage, 5-year survival rate of HCC has been reported to be less than 10%, regardless of the treatment modalities.2-4 Recently, early diagnosis of HCC is on the rise because of regular surveillance in high risk groups. If detected early, HCC could be completely cured by surgical resection or liver transplantation.5,6 Nevertheless, if HCC recurs after such curative treatments, the survival rate of patients becomes noticeably low.2,7 It has been reported that vascular infiltration of tumors has been reported to be an risk factor for recurrence of HCC.8,9 The mechanism of vascular invasion of tumors has not been completely characterized, although recent studies reported that the process of epithelial mesenchymal transition might be associated with cancer metastasis.10,11 It appears that cells transformed from epithelial cells to interstitial cells are connected loosely, and thus they could migrate readily, invasiveness becomes high, invade blood vessels and metastasized to other organs.
Snail, first identified in drosophila, plays an important role in the mesenchymal formation.12 Snail is a transcription factor containing the helix-loop-helix structure, and it has been reported to suppress the transcription of E-cadherin, induce the expression of matrix metalloproteniase-2, and thus degrade extracellular matrix.13-15 In previous reports on the expression of Snail mRNA in HCC tissues, the expression of Snail has been shown to be significantly associated with the histological grade of differentiation of HCC, portal venous invasion and intrahepatic recurrence.16,17 Nonetheless, such studies were conducted on HCC primarily associated with hepatitis C virus, and results of the studies on HCC associated with hepatitis B virus are known to be rare. We examined the expression of Snail and E-cadherin in resected tissues of HCC patients whose etiology was hepatitis B in most cases, and its relationship with pathophysiological characteristic of HCC and clinical outcomes of patients.
The study subjects were 59 HCC patients performed surgical resection or liver transplantation for HCC from 1997 to 2004 at our hospital. First, the expression of Snail and E-cadherin in tissues obtained after surgery embedded in paraffin blocks was assessed by immunohistochemical analysis. After that, by using the medical record of subject patients, followings were examined; baseline clinical features, clinical courses after surgery, histological grade of differentiation, tumor stage, and the presence or absence of hepatic portal venous invasion. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. This study was also approved by Kangnam St. Mary's Hospital Institutional Review Board.
Immunohistochemical staining was performed on surgical specimen of the 59 HCC patients prepared as paraffin blocks. Briefly, the samples were treated with xylene to remove paraffin, and added to low concentrations of alcohol sequentially. The sections were added to methanol containing 3% hydrogen peroxide, and blocked with phosphate buffered saline containing 1% Bovine serum albumin. Then after the sections were reacted with rabbit polyclonal Snail antibody (DECMA-1, Cambridge, UK) diluted to 1:600 with phosphate buffered saline at 4℃ for 24 hours and rat monoclonal E-cadherin antibody (DECMA-1, Cambridge, UK) diluted to 1:500. Hydrogen peroxide was removed with the EnVision System, and kept at room temperature for 10 minutes. It was stained with 3,3'-diaminobenzidine tetrachloride, washed, and as counterstaining, haematoxylin was used.
To assess the staining of Snail and E-cadherin, tissue samples were examined at 200 times magnification, and classified as follows. Samples with more than 10% stained tumor cells were divided to 3 grades according to staining intensity and read. For Snail staining, samples with a portion of tumor cells not clearly stained were read as 1, samples with the nucleus of a portion of tumor cells or the entire tumor cells stained at moderate intensity were 2, and samples with the nucleus of a portion of tumor cells or the entire tumor cells stained strongly were 3. For E-cadherin staining, samples with the stained cytoplasm was 1, weak staining of cell membrane of a portion of tumor cells or the entire tumor cells was 2, and the noticeable staining of cell membrane of a portion of tumor cells or the entire cells was 3. For statistical analysis, no staining or staining 1 were considered as negative, and staining 2 and 3 were considered as positive.
As clinical factors, the age of patients, gender, the cause of HCC, serum alpha-fetoprotein level, tumor stage, the size of HCC, number, location, and morphological type were analyzed. The disease stage of tumor disease was evaluated according to the modified 5th UICC classification based on the primary liver cancer code of the Korean liver cancer research society.18 Pathophysiologically, the presence or absence of portal venous invasion of HCC and the histological differentiation grade classified according to the Edmondson-Steiner's grade I, II, III, and IV19 was analyzed in association with the expression of Snail and E-cadherin.
To evaluate the association of the expression of Snail and E-cadherin to clinical and pathological factors of HCC, Fisher's exact test or Mann-Whitney's U-test was used as appropriately. The postsurgical recurrence rate of the Snail positive and negative group, and that of the E-cadherin positive and negative group were compared applying Kaplan-Meier survival analysis and log-rank test. Statistical significance of the result of analysis was determined by P-value<0.05. All statistical analysis was performed by the SPSS version 12.0 (SPSS Inc, Chicago, IL, USA).
The mean age of the 59 patients was 57 years (range, 35-80 years). Forty eight patients was male (81.4%). As the cause of HCC, 43 cases was hepatitis B (72.9%), which was most prevalent, and hepatitis C was 8 cases (13.6%). Among the all patients, 34 patients received surgical resection, and 25 patients received liver transplantation. The mean score of the Model for End Stage Liver Disease at the time of the radical treatment was 10.8 (range, 6-30). The median serum alpha-fetoprotein was 161.5 ng/mL (range, 1.26-2,907 ng/mL). In regard to the HCC stage, 1 case (1.7%) was stage 1, 35 cases (59.3%) was stage 2, 9 cases (15.3%) was stage 3, and 14 cases (23.7%) was stage 4. The mean diameter of tumor was 5 cm, 40 cases (67.8%) were smaller than 5 cm, and 19 cases (32.2%) were larger than 5 cm. The tumor was solitary in 71.2%. Regarding the morphological type of tumors, 56 cases (94.9%) was nodular type, 1 case (1.7%) was infiltrative type, and 2 cases (3.4%) was multinodular type. Concerning the histological differentiation grade of HCC, grade I/II/III/IV was 7/18/22/12, respectively, and cases with portal vein invasion were 14 patients (23.7%) (Table 1).
Snail was expressed in the HCC in 12 cases (20.3%) and in the adjacent non-HCC in 2 cases (3.4%). E-cadherin was expressed in the cytoplasmic membrane of HCC in 26 cases (44.1%). In addition, in all cases, in the HCC area expressing Snail, the expression of E-cadherin was suppressed (Fig. 1).
The expression of Snail according to the histological grade of differentiation in HCC was 0 case (0%) in grade I, 3 cases (16.7%) in grade II, 3 cases (13.6%) in grade III, and 6 cases (50%) in grade IV, respectively (P=0.028). In addition, regarding the expression of Snail according to the portal vein invasion, it was expressed in 7 cases without portal vein invasion (15.5%), and 5 cases with the invasion (35.7%). Although the expression was increased in cases with portal vein invasion, it was not statistically significant (P=0.102). The etiology of HCC, tumor stage, the size, number and morphological type of HCC were not significantly associated with the expression of Snail and E-cadherin (P>0.05) (Table 2). When the clinicopathological factors according to the expression of E-cadherin in the group with or without Snail expression were compared, in the group with Snail expression and the group without the expression, clinicopathological factors were not significantly different according to the expression of E-cadherin (Table 3).
During the average 33 months of follow-up period (range, 1-133 months) after operation, HCC recurred in 29 patients (49.1%) among the entire 59 patients. Among them, 16 cases (27.1%) was intrahepatic recurrence, 19 cases (32.2%) was extrahepatic metastasis, and intrahepatic recurrence and extrahepatic metastasis simultaneously occurred in 6 cases (10.1%).
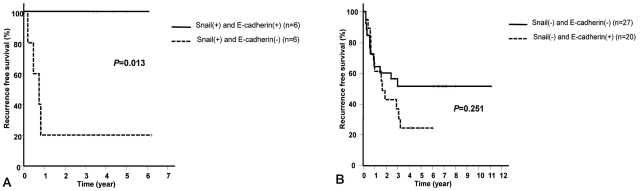
HCC recurrence rate and survival rate after resection was not different according to the expression of Snail and E-cadherin. Nonetheless, in patients expressing Snail without the expression of E-cadherin, the recurrence rate of HCC after surgery was significantly higher than patients expressing both Snail and E-cadherin (P=0.013) (Fig. 2A). Meanwhile, in patients without Snail expression, HCC recurrence rate was not significantly different depending on the presence or absence of the expression of E-cadherin (P=0.251) (Fig. 2B).
It has been shown that E-cadherin plays an important role in mediating cell to cell adhesion,20 and the low expression of E-cadherin is closely associated with local invasion of tumor cells and distant metastasis.20, 21 Several mechanisms controlling the expression of E-cadherin have been shown, and recently, Snail gene has been reported to be a mechanism suppressing E-cadherin. Snail is a gene discovered in Drosophila for the first time. It has been shown to play an essential role in the formation of the mesenchyme because Drosophila with the mutation in Snail could not form the mesenchyme and thus die in the embryonic period.12,22 In addition, it has been reported that Snail suppress E-cadherin directly, accelerates the expression of matrix metalloproteinase, and thus involved in the transition of epithelial cells to mesenchymal cells as the important stage of metastasis.13-15 In recent studies, Snail has been reported to be overexpressed in 16-23% of HCC tissues, and expressed inversely to E-cadherin.15,17,23 In our study, Snail was expressed in the HCC area of 20.3% of cases (12/59) and in the adjacent non-HCC area in 3.4% of cases (2/59). E-cadherin was expressed in 44.1% of HCC area (26/59), however, the expression of E-cadherin in the non-HCC area was not examined additionally. However, according to studies reported previously, E-cadherin has been shown to be highly expressed in the non-HCC area in more than 90% of cases.24 In addition, in our study, in all cases, the expression of E-cadherin was suppressed in the HCC area expressing Snail. Based on these results, in Korea where the major etiology is hepatitis B, Snail was overexpressed in HCC as comparable frequencies to other studies, and also, it was expressed inversely to E-cadherin.
It has been shown that Snail not only suppresses E-cadherin but also accelerates the infiltration of tumor cells in various malignant tumors.25,26 The expression of Snail in HCC is associated with poorly differentiated histological grade, with capsular and vascular invasion, and related to post surgical intrahepatic recurrence.15-17 In our study, in the association of the expression of Snail with clinicopathological characteristics, the histological grade of differentiation in HCC expressing Snail was poor with statistical significance. However, in regard to vascular invasion, although portal vein invasion was more frequent in HCC expressing Snail, it was not statistically significant. It has been shown that histological grade of differentiation and vascular invasion are closely related to the invasion and metastasis in HCC, and thus it could be predicted that the prognosis of HCC expressing Snail would be poorer than HCC without its expression. On the other hand, E-cadherin has been shown to be closely associated with the differentiation grade of HCC, and as the differentiation grade of primary HCC was poorer, the rate of the loss of the expression was high.27 However, in this study, E-cadherin did not show the significant association with any clinicopathological factors. Such results may be due to the insufficient number of included patients and the inhomogeneous subject patients in each study.
In our study, the expression of Snail or E-cadherin itself did not show the association with the recurrence rate of tumors after resection, although, in HCC expressing Snail without expressing E-cadherin, the recurrence rate after surgery was shown to be significantly higher than in HCC cases expressing both Snail and E-cadherin. Based on the results, it could be speculated that the expression of Snail was associated with the suppression of the expression of E-cadherin as well as the recurrence after surgical treatment. However, HCC expressing E-cadherin despite of the expression of Snail was present, and in such cases, poor prognosis as in the cases of HCC without expressing E-cadherin was not shown, and thus it is difficult to predict the effect on the postsurgical recurrence rate by the simple expression pattern of Snail and E-cadherin. In addition, the possibility that mechanisms other than Snail may be involved in the action mechanisms of E-cadherin could not be ruled out. In previous reports, it has been reported that in the mechanism of the regulation of E-cadherin, in addition to Snail, the hypermethylation of E-cadherin gene or the gene regulation area, or the loss of heterozygosity is involved in it.28-30
In conclusion, Snail was expressed in approximately 20% of HCC cases. E-cadherin was also expressed in HCC, and the expression was suppressed in the area expressing Snail. The expression of Snail was significantly increased in HCC with poor histological differentiation. Further studies on the association of the Snail expression with the recurrence of tumor after surgery would be needed.
Acknowledgements
This study was co-supported by the 2010 clinical research fund of the Busan University Hospital and the grant (A050021, Clinical Research Center for Liver Cirrhosis) from the Good Health R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
REFERENCES
1. Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat 2004;36:103-114. 20396549.




2. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24. 10862190.



3. Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg 1996;131:71-76. 8546582.


4. Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer 1998;82:1028-1036. 9506346.


5. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918-928. 2990661.


6. Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999;229:322-330. 10077043.



7. Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999;229:790-799. 10363892.



8. Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, Blumgart L, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995;169:28-34. 7817995.


9. Shimada M, Hasegawa H, Gion T, Shirabe K, Taguchi K, Takenaka K, et al. Risk factors of the recurrence of hepatocellular carcinoma originating from residual cancer cells after hepatectomy. Hepatogastroenterology 1999;46:2469-2475. 10522022.

10. Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer 1996;77:2022-2031. 8640665.


11. Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, et al. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 2002;115:1189-1202. 11884518.

12. Grau Y, Carteret C, Simpson P. Mutations and Chromosomal Rearrangements Affecting the Expression of Snail, a Gene Involved in Embryonic Patterning in DROSOPHILA MELANOGASTER. Genetics 1984;108:347-360. 17246230.




13. Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76-83. 10655586.


14. Olmeda D, Jordá M, Peinado H, Fabra A, Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007;26:1862-1874. 17043660.


15. Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K, et al. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer 2005;92:252-258. 15668718.



16. Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 2002;21:3241-3246. 12082640.


17. Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res 2003;9:2657-2664. 12855644.

18. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002;24:395-403. 12479938.


19. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503. 13160935.


20. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991;251:1451-1455. 2006419.


21. Endo K, Ueda T, Ueyama J, Ohta T, Terada T. Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum Pathol 2000;31:558-565. 10836294.


22. Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development 1990;110:73-84. 2081472.



23. Jiao W, Miyazaki K, Kitajima Y. Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer 2002;86:98-101. 11857019.



24. Bae SH, Jung ES, Park YM, Jang JW, Choi JY, Cho SH, et al. Expression patterns of E-cadherin and beta-catenin according to clinicopathological characteristics of hepatocellular carcinoma. Korean J Hepatol 2002;8:297-303.
25. Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000;2:84-89. 10655587.


26. Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol 2001;37:65-71. 11120485.


27. Ihara A, Koizumi H, Hashizume R, Uchikoshi T. Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology 1996;23:1441-1447. 8675162.


28. Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology 2002;36:692-701. 12198663.


Figure 1
Immunohistochemical staining patterns of Snail and E-cadherin in hepatocellular carcinomas (HCCs). (A) Positive staining of Snail is seen in the tumor portion. (B) E-cadherin is not stained in the tumor portion where they show positive staining for Snail. (C) Positive staining of E-cadherin is noted in the cytomembrane of the tumor portion. (D) Snail is not stained in the tumor portion where they show positive staining for E-cadherin (A: Snail, ×400, B: E-cadherin, ×400, C: E-cadherin, ×400, D: Snail, ×400).
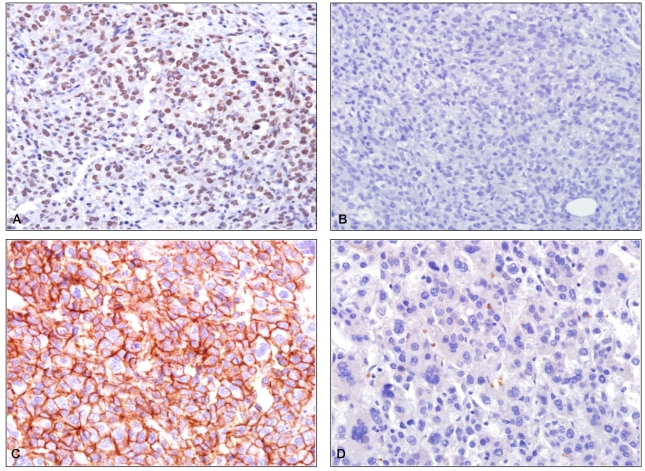
Figure 2
Cumulative recurrence-free survival rate of HCC according to immunohistochemical staining patterns of Snail and E-cadherin (Kaplan-Meier survival curve, P-value by log-rank test). (A) The cumulative recurrence-free survival rate of HCC is significantly higher in negative E-cadherin staining than in positive E-cadherin staining in the case of positive Snail staining. (B) The cumulative recurrence-free survival rate of HCC does not differ significantly according to the E-cadherin staining pattern in the case of negative Snail staining.
- TOOLS
-
METRICS

- Related articles
-
Recent advances in the management of hepatocellular carcinoma2024 January;30(1)






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



