| Clin Mol Hepatol > Volume 21(3); 2015 > Article |
Cholangiocarcinoma (CC) is an adenocarcinoma arising from epithelial cells of varying locations within the biliary tree, and is the second most common primary liver tumor, accounting for approximately 10-15% of all hepatobiliary malignancies.1 This disease has a high prevalence in Southeast and Eastern Asia, including Korea; during the period 2003-2005, the age-standardized incidence rates of intrahepatic CC and extrahepatic CC were reported to be 6.9 and 5.2 out of 100,000 in Korea, respectively.2 Sarcomatoid carcinoma is a rare tumor composed of mixed malignant epithelial and mesenchymal cells, and it can occur in various organs including liver. The prevalence of sarcomatoid CC has been reported to be about 4.5% of surgical and autopsied cases.3 Most sarcomatoid CCs have been reported to have sarcomatous component which show spindle cell or pleomorphic cell differentiation.4 Furthermore, sarcomatoid CC with osteoclast-like giant cells, which morphologically resemble those found in giant cell tumors of the bone is a very rare malignant liver tumor which was first reported as osteoclastoma-like giant cell tumor of the liver by Munoz et al. in 1980.5 To the best of our knowledge, sarcomatoid CC with osteoclast-like giant cells are extremely rare and only 5 cases were reported in the literature so far.6 Here we report a case of sarcomatoid CC with osteoclast-like giant cells, which is associated with hepatolithiasis.
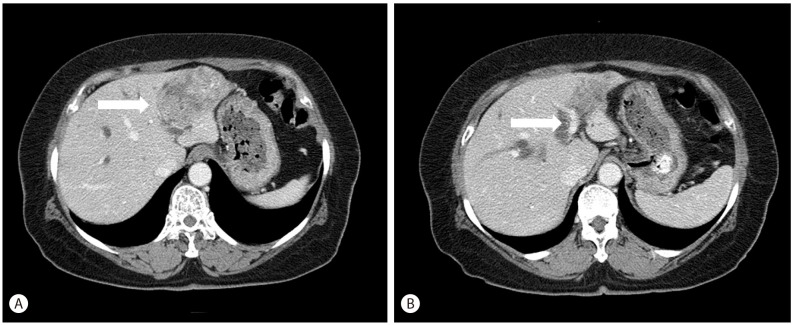
A 67-year-old woman was referred to our hospital for right upper quadrant abdominal pain for 3 weeks. She had no history of alcohol ingestion or smoking, and no remarkable medical history. A computed tomography scan was performed to evaluate the cause of abdominal pain, and revealed distal common bile duct stone or sludge with dilatation of the common bile duct and bilateral intrahepatic ducts and a 6 cm-sized heterogeneous lobulated mass in the left lateral segment of the liver, suggestive of intrahepatic CC with multiple left intrahepatic duct stones (Fig. 1A, B). There was no definite evidence of hepatic artery or portal vein invasion or intrahepatic metastasis. Initial laboratory findings showed normal liver function tests: aspartate aminotransferase 16 IU/L, alanine aminotransferase 22 IU/L, total bilirubin 0.4 mg/dL, direct bilirubin 0.1 mg/dL and elevated gamma-glutamyl transpeptidase 97 IU/L and alkaline phosphatase 151 IU/L. Tumor marker tests revealed elevated carbohydrate antigen 19-9 (1598.0 U/mL) and carcinoembryonic antigen (109.60 ng/mL) levels. Testing for hepatitis B and hepatitis C virus were negative. Under the impression of mass-forming intrahepatic CC, she underwent a left lobectomy of the liver.
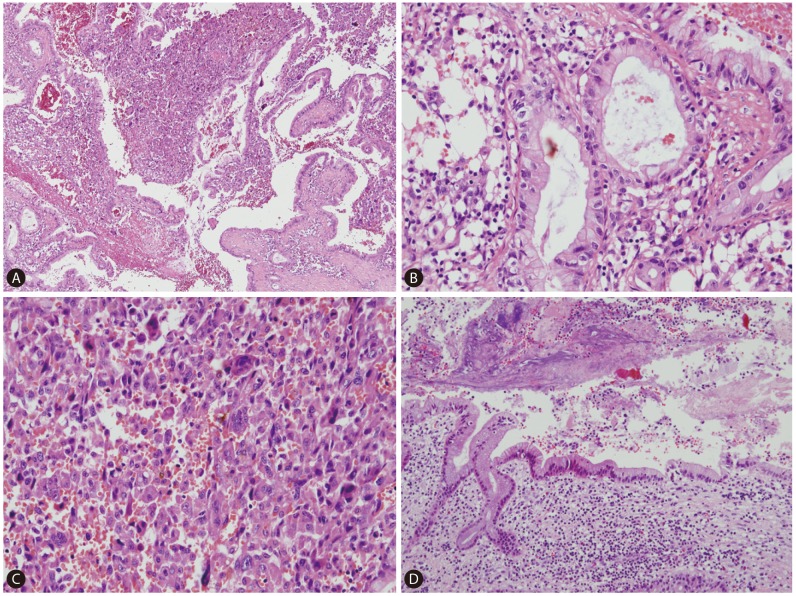
On gross examination, the cut surface revealed a single, firm, whitish to tan mass (4.5├Ś4.0 cm) with periductal infiltration around the dilated left intrahepatic duct. The duct lumen was distended to about 2 cm in diameter with 3 cm sized black stone impaction (Fig. 2). Microscopically, the tumor showed two distinct components; carcinomatous component and sarcomatous component (Fig. 3A, 4A). In the carcinomatous component, the malignant cells formed irregular papillae or fused glands in a prominent fibrous stroma, consistent with features of a typical well-to-moderately differentiated adenocarcinoma. The columnar-to-cuboidal epithelial neoplastic cells had atypical nuclei with some prominent nucleoli, slightly eosinophilic and granular cytoplasm, and some mitosis (Fig. 3B). In the sarcomatous component, there were pleomorphic cells and atypical spindle cells with vesicular nuclei and prominent nucleoli (Fig. 3C). The two components were intermingled. In addition, there were many scattered osteoclast-like giant cells: large multinucleated giant cells containing 10-25 nuclei and about 150-200 ┬Ąm in diameter (Fig. 4F). Perineural invasion and microvessel invasion were seen. In surrounding liver, hepatolithiasis with chronic proliferative cholangitis and microabscess formation was noted (Fig. 3D). The immunohistochemical staining results also demonstrated two distinct patterns. The carcinomatous portion was positive for cytokeratin 19 (Fig. 4B) and negative for vimentin. The sarcomatous portion was positive for vimentin (Fig. 4E) and negative for cytokeratin 19. Both were negative for hepatocyte antigen and alpha-fetoprotein. The osteoclast-like giant cells were positive for CD68 (PG-M1) (Fig. 4F). The immunohistochemical staining with EMT-related markers, E-cadherin and uPAR was conducted. E-cadherin showed total loss in the sarcomatous portion and no loss in the carcinomatous portion (Fig. 4C). uPAR was positive in the sarcomatous portion and peritumoral stroma but negative in the carcinomatous portion (Fig. 4D).
Sarcomatoid CC is an extremely rare liver primary tumor, and its pathogenesis still remains unclear. Sarcomatous change may be explained by epithelial-mesenchymal transition (EMT), which refers to the biologic process of epithelial phenotype changing to mesenchymal phenotype by undergoing multiple biochemical changes including enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and increased production of extracellular matrix components. A hallmark of EMT is the loss of epithelial characteristics such as a decrease in the expression of the cell adhesion molecule E-cadherin and acquisition of a mesenchymal phenotype accompanied by increased expression of vimentin. Transforming growth factor ╬▓ as well as transcription factors such as Twist, Snail, Slug, Sip1, ZEB1 and ZEB2 have a regulatory role in EMT.7 Also, increased uPAR expression has been implicated in the promotion of EMT in various cancers.8 Previous report about 27 cases of sarcomatoid carcinoma originating from various organs, mostly from gastrointestinal tract which included 4 cases of CCs demonstrated that most sarcomatoid carcinomas express EMT-related markers such as Twist1, Snail1, Slug, Sip1 and ZEB1.9 Presenting case showed a positive expression for EMT-related markers: vimentin, uPAR, and loss of E-cadherin, suggesting of EMT.
Another sarcomatoid carcinoma primarily occurring in the liver is sarcomatoid hepatocellular carcinoma (HCC), and 73 cases of sarcomatoid HCC have been reported in the English literature in the past 20 years.10 Preoperative anticancer treatment such as transcatheter arterial chemoembolization, radiofrequency ablation or percutaneous ethanol injection has been reported to be associated with sarcomatous change in HCC.11 Sarcomatoid HCC without previous therapies was also reported, although there are only a few reports of such cases.12 The present patient with sarcomatoid CC had no history of preoperative treatment, therefore, preoperative treatment is not considered to be necessary for the sarcomatoid change.
Interestingly, this case showed osteoclast-like giant cells in addition to spindle cells of sarcomatous component. Tumors that contain osteoclast-like giant cells have been reported in several sites, which include the pancreas, lung, thyroid gland, stomach, breast, uterus, kidney, soft tissue, salivary glands,13 and liver.14 Osteoclast-like giant cells which closely resemble those of giant cell tumors of bone may be derived from bone marrow monocytes as they express the monocytic/histiocytic marker, CD6815 and are negative for the epithelial markers. In this case, they were positive for CD68, and were negative for the epithelial marker, cytokeratin 19. These findings support that osteoclast-like giant cells are not epithelial origin but monocytic/histiocytic origin.
This presenting case showed chronic cholangitis with hepatolithiasis in the non-neoplastic liver. Clinically, hepatolithiasis is considered as a main cause of CC in Korea16 and approximately 10% of patients with hepatolithiasis develop CC.17 Repeated episodes of cholangitis due to hepatolithiasis are considered to induce proliferative epithelial changes and accelerate tumorigenesis.18
The prognosis of CC is generally unfavorable because of frequent metastasis, low resectability rate and high incidence of recurrence even after curative resection. The median survival is 27 months, and 5-year survival was 31%.19 The outcome of sarcomatoid CC appears to be even worse than conventional CC without sarcomatous change.20 Previous reports have demonstrated that these tumors are consistently very aggressive because of intrahepatic metastasis and frequent widespread metastasis of sarcomatoid cells.3 Therefore, early detection and complete surgical resection might be the only chance to improve the patient's outcome.21 The present case had no intrahepatic metastasis or extrahepatic metastasis at the time of operation, and there was no evidence of cancer recurrence at 6 months after operation.
REFERENCES
1. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:221-232. 25966423.


2. Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci 2010;25:1011-1016. 20592891.



3. Nakajima T, Tajima Y, Sugano I, Nagao K, Kondo Y, Wada K. Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer 1993;72:1872-1877. 7689920.


4. Malhotra S, Wood J, Mansy T, Singh R, Zaitoun A, Madhusudan S. Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol 2010;2010:701476. 20454704.




5. Munoz PA, Rao MS, Reddy JK. Osteoclastoma-like giant cell tumor of the liver. Cancer 1980;46:771-779. 6994872.


6. Dahm HH. Immunohistochemical evaluation of a sarcomatoid hepatocellular carcinoma with osteoclastlike giant cells. Diagn Pathol 2015;10:40. 25928039.



7. Garg M. Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J Stem Cells 2013;5:188-195. 24179606.



8. Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol 2007;178:425-436. 17664334.



9. Sung CO, Choi H, Lee KW, Kim SH. Sarcomatoid carcinoma represents a complete phenotype with various pathways of epithelial mesenchymal transition. J Clin Pathol 2013;66:601-606. 23533257.


10. Giunchi F, Vasuri F, Baldin P, Rosini F, Corti B, D'Errico-Grigioni A. Primary liver sarcomatous carcinoma: report of two cases and review of the literature. Pathol Res Pract 2013;209:249-254. 23484778.


11. Chin S, Kim Z. Sarcomatoid combined hepatocellular-cholangiocarcinoma: a case report and review of literature. Int J Clin Exp Pathol 2014;7:8290-8294. 25550887.


12. Yokomizo J, Cho A, Yamamoto H, Nagata M, Takiguchi N, Kainuma O, et al. Sarcomatous hepatocellular carcinoma without previous anticancer therapy. J Hepatobiliary Pancreat Surg 2007;14:324-327. 17520211.


13. Rosai J. Liver cell carcinoma with osteoclast-like giant cells: nonepitheliogenic giant cells in diverse malignancies. Hepatology 1990;12:782-783. 2170268.


14. Lee KB. Sarcomatoid hepatocellular carcinoma with mixed osteoclast-like giant cells and chondroid differentiation. Clin Mol Hepatol 2014;20:313-316. 25320737.



15. Gao HQ, Yang YM, Zhuang Y, Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol 2015;21:694-698. 25593500.



16. Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716-1720. 18557716.


17. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-184. 21488076.



18. Haratake J, Yamada H, Horie A, Inokuma T. Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer 1992;69:2444-2448. 1314689.


19. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-3145. 21730269.


Figure┬Ā1
Image finding. An axial CT image shows a 6cm-sized heterogeneous lobulated mass in the left lateral segment of the liver (A). Another CT image shows bilateral intrahepatic duct dilatation and multiple left intrahepatic duct stones, measuring 3 cm in the largest one (B).
Figure┬Ā2
Gross finding. The representative cut surface reveals a single, firm, whitish to tan mass (4.5├Ś4.0 cm) with periductal infiltration around the dilated left intrahepatic duct. The lumen of bile duct is distended up to 2 cm in diameter and impacted with pigment stone.

Figure┬Ā3
Microscopic finding. Carcinomatous component and sarcomatous component are intermingled (A. H-E, ├Ś40). Carcinomatous component shows the features of typical well-to-moderately differentiated adenocarcinoma (B. H-E, ├Ś200). Sarcomatous component is consisted with pleomorphic cells and atypical spindle cells (C. H-E, ├Ś200). In surrounding liver, hepatolithiasis with chronic proliferative cholangitis and microabscess formation are noted (D. H-E, ├Ś100).
Figure┬Ā4
Immunohistochemical finding. Microscopic feature of sarcomatoid cholangiocarcinoma shows intermingled carcinomatous and sarcomatous component (A. H-E, ├Ś100). Carcinomatous component shows strong expression of cytokeratin 19, but sarcomatous component shows no expression of cytokeratin 19 (B. CK19, ├Ś200). E-cadherin shows total loss in the sarcomatous portion and no loss in the carcinomatous portion (C. E-cadherin, ├Ś200). uPAR is positive in the sarcomatous portion but negative in the carcinomatous portion (D. uPAR, ├Ś200). Sarcomatous component shows diffuse and strong expression of vimentin (E. vimentin, ├Ś200). Many scattered osteoclast-like giant cells shows positive expression of CD68 (PG-M1) (F. H-E and CD68 (PG-M1), ├Ś400).

- TOOLS
-
METRICS

- Related articles
-
Intrahepatic cholangiocarcinoma with predominant ductal plate malformation pattern2014 June;20(2)
Intrahepatic cholangiocarcinoma associated with Clonorchis sinensis infection2009 December;15(4)
Intrahepatic Cholangiocarcinoma associated with Hepatolithiasis2007 September;13(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



