| Clin Mol Hepatol > Volume 18(4); 2012 > Article |
ABSTRACT
Background/Aims
Sustained virologic response (SVR) for the treatment of chronic hepatitis C (CHC) may differ with ethnicity due to differences in genetic traits. This study evaluated the efficacy of peginterferon and ribavirin, and the association between IL28B genotypes and the treatment efficacy in Korean CHC patients.
Methods
This was a retrospective cohort study using data from medical records. Eighty-five CHC patients were eligible for assessment of the efficacy of antiviral therapy, and 47 patients were available for an IL28B genetic study, which was performed using the Multiplex tetra-primer PCR method for rs12979860.
Results
Overall, the early virologic response rate was 87.1%: 84.9% in HCV genotype 1 and 90.6% in genotype 2. The overall end-of-treatment virologic response rate was 81.2%: 75.5% in genotype 1 and 90.6% in genotype 2. The overall SVR rate was 81.2%: 75.5% in genotype 1 and 90.6% in genotype 2. For rs12979860, the frequencies of polymorphisms were 89% for the CC type, 11% for the CT type, and 0% for the TT type. Their overall SVR rate was 87% (39/47): 90.5% (38/42) for the CC type and 20% (1/5) for the CT type. For genotype 1, SVR rates were 88% (21/24) for the CC type and 0% (0/4) for the CT type. Multivariate analysis revealed that the IL28B-CC type was a good predictor for SVR.
It is expected that there are approximately 170 million hepatitis C virus (HCV) infected individuals in the world.1 In Korea, 1-2% of population is infected, and 15-20% of them have chronic liver diseases from HCV infection.2 On an annual basis, 3-4 million people are infected through blood transfusion, reckless drug abuse, sexual contact, and exposure during childbirth. Symptoms are rarely shown during the initial infection stage, but 55-85% of all HCV-infected individuals develops CHC. Because 20-30% of all HCV-infected individuals develop liver cirrhosis and 1-4% of all patients with liver cirrhosis develops hepatocellular carcinomas, hepatitis C is rising as a grave public health and medical issue in the world.3-9 It has been reported that SVRs of Asian patients are higher than in Western countries. It has been thought due to the difference of genetic traits.
Recent genome-wide association studies have showed that several single nucleotide polymorphisms (SNPs) near IL28B were associated with favorable treatment response in patients infected with genotype 1 HCV and natural viral clearance.10-12 In addition, it has been reported that genetic variation of IL28B is associated with SVR according to ethnities.13 Therefore, we investigated treatment efficacy of pegylated interferon and ribavirin combination therapy, genetic variation of IL28B in CHC patients, and treatment response of CHC patients according to IL28B polymorphisms.
The research was conducted subject to 103 patients who had been hospitalized and treated for the chronic hepatitis C infection at the Gachon university Gil hospital from July 2004 to December 2009. Among these research subjects, a retrospective cohort study was carried out subject to 85 patients who had been treated with a combination therapy using peginterferon alfa-2a and ribavirin.
The criteria for selecting a patient includes those who were over or at age of 18 as well as those who had been detected of anti-HCV and HCV RNA within the serum. Subjects with hemoglobin <10 g/dL, neutrophils <750/mm3, platelets <5.0×103/mm3, renal dysfunction (serum creatinine >1.4 mg/dL), decompensated liver-function disorder, a serious high blood-pressure, heart failure, a serious coronary artery disease, uncontrollable diabetes, obstructive pulmonary disease, a personal history of chronic drinking and drug overdose, an autoimmune hepatitis, suspected hepatocellular carcinoma, thyroid dysfunction, either pregnant or not suitable for a regular contraception, hypersensitivity towards drugs, and a serious psychological disease were excluded from the combination therapy group.
The definition of liver cirrhosis was defined as a condition where there is a nodular change of liver during the ultrasonography and a condition where there is an esophageal varix identified by an endoscopy. In addition, a diagnosis of the portal hypertension such as splenomegaly or a condition in which the blood platelet count is lower or equal to 80,000/mm3 are also included in the definition of a liver cirrhosis. A definition of compensated liver cirrhosis was defined as liver cirrhosis without any clinical evidence or a medical history of liver cirrhosis complications such as ascites, icterus, hepatorenal syndrome, variceal hemorrhage and etc. In case there is a clinical evidence for above-mentioned medical histories, the condition was defined as decompensated liver cirrhosis. We have investigated the effects and the safety of the combination therapy subject to patients who are treated with a combination therapy utilized by peginterferon and ribavirin for the treatment of CHC at Gachon University Gil Hospital.
Among treated CHC patients, we had searched some patients who agreed to DNA sequencing of IL28B. The clinical study was approved by local ethics committees and written informed consent for DNA sequencing was obtained. This study was done with provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Chemilumino immuno assay was used to investigate into Anti-HCV, COBAS AmpliPrep™ (Roche, IN, USA) into a qualitative test of HCV RNA, and Polymerase Chain Reaction (PCR) using Abbott m2000rt instrument (Abbott GmbH & Co. KG, Wiesbaden, Germany) was employed to conduct a quantitative test of HCV RNA. HCV genotype was amplified using core region of HCV cDNA as PCR Method after which its DNA sequence was acquired through the Sequencing Method. Last but not least, a research was conducted using ABI 3130XL Genetic Analyzer, Applied Biosystems through searching HCV genotype blast.
Patients with genotype-1 Chronic HCV infection were injected 180 µg of peginterferon alpha 2a (Pegasys®, F. Hoffmann-La Roche Ltd, Basel, Switzerland) per week regardless of weight and 1,200 mg of ribavirin per day for patients over 75 kg and 1,000 mg of ribavirin per day for below 75 kg. The treatment lasted for 48 weeks. Patients with non-1 genotype chronic HCV infection were injected 180 µg of peginterferon alpha 2a per week and 800mg of ribavirin per day for 24 weeks. Four weeks before all treatments, blood test, liver function test, and HCV RNA conditioning test were carried out. Injection lasted 48 weeks for genotype 1 and 24 weeks for genotype non-1. A follow up test including blood test, liver function test, and HCV RNA conditioning test is carried out every 4-12 weeks after treatment. At the end of and 24 weeks after treatment, a liver function test and HCV RNA conditioning test were conducted. The reduction of peginterferon was implemented in a stage by 75% and 50% levels and when absolute neutrophil count were less than 750/mm3 and the number of thrombocyte less than 30,000/mm3, injection came to a halt. As for a ribavirin, if hemoglobin decreased to lower than 10 g/dL, a dose was reduced by stage to 800 mg and 600 mg and when it marked less than 8 g/dL, treatment came to a halt.
Early virologic response (EVR) was defined as the occurrence of virus negativity or reduction in more than 2log of HCV RNA in a quantitative test after 12 weeks of treatment received by patients who have undergone HCV qualitative or quantitative tests. The end of treatment virologic response (ETVR) was defined as the negativity of HCV RNA after the end of treatment, while SVR as the continuation of ETVR even 24 weeks after the end of treatment.
Blood was collected into EDTA tubes. Genomic DNA was extracted from whole blood with the QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA quality was assessed by calculating the absorbance ratio OD260 nm/280 nm using NanoDrop model ND-1000 (Thermo Fisher Scientific Inc., Wilmington, USA) and DNA quantity was estimated by the Quibit® dsDNA BR Assay kit using Quibit® Fluorometer (Invitrogen, California, USA). IL28B rs12979860 Genotyping was detected by Multiplex tetra-primer PCR method. PCR reaction was performed with 1 µL whole blood, all 4 primers (Con F: GCTCAGCGCCTCTTCCTCCT-3, Con R: TCCCATACACCCGTTCCTGT-3, IL28 (T) F, 5-AGGAGCTCCCCGAAGGAGT-3, IL28 (C) R: TGCAATTCAACCCTGGTACG-3), EzWay™ Direct Taq PCR MasterMix (Koma Biotech Inc., Seoul, Korea) in 20 µL reaction volume. The amplification was carried out with an enzyme activation step for 5 min at 95℃, followed by 35 cycles of 45 s at 95℃, 45 s at 60℃, and 45 s at 72℃, and a final extension for 10 min at 72℃. The amplicons were separated by 2% agarose gel electrophoresis and stained with ethidium bromide (0.5 mg/L). Both homozygous (CC and TT) and heterozygous genotypes (CT) were easily detected on agarose gel.
A statistical analysis was conducted using SPSS version 15.0. Mann-Whitney U-test was used for comparison between continuous variables' mean values and Fisher's exact test for that between categorical variables. Univariate analysis was implemented to understand factors affecting SVR while a multivariate analysis using a logistic regression model was conducted to analyze independent factors. A P-value less than or equal to 0.05 was considered statistically significant.
Baseline data are shown in Table 1. Among 85 patients, 53 were genotype 1, 32 were genotype 2 (31 in genotype 2a and 1 in genotype 2b). Six patients were dropped out because of side effects and follow-up loss.
EVR rates were 84.9% (45/53) in genotype 1 and 90.6% (29/32) in genotype 2. ETVR rates were 75.7% (40/53) in genotype 1 and 90.6% (29/32) in genotype 2. SVR rates were 81.2% (69/85); 75.5% (40/53) in genotype 1 and 90.6% (29/32) in genotype 2. For 79 patients followed up to 6 months after completion of treatment, SVR rates were 81.6% in genotype 1 and 96.7% in genotype 2 (Fig. 1).
On univariate analysis, HCV genotype (P=0.034), dose of peginterferon (P=0.016), platelet count (P=0.015), serum ALT level (P=0.003) and EVR (P=0.001) were associated with SVR (Table 2). On multivariate analysis, dose of peginterferon (≥75%, OR=4.965, 95% CI; 1.001-24.631, P=0.050), HCV genotype (genotype 1, OR=0.067, 95% CI; 0.006-0.798, P=0.032), and EVR (OR=27.634, 95% CI; 3.753-203.487, P=0.001) were independent predicting factors (Table 3).
Various side effects were observed during the treatment (Table 4). Common laboratory side effects were hematologic like anemia (31.8%), neutropenia (22.4%), and thrombocytopenia (17.7%). General weakness (27.1%), myalgia (24.7%), and itching(21.2%) were also common. Besides, hypothyroidism (3.5%), vomiting (2.4%), and rash (1.2%) were observed (Table 4).
Among 85 patients, 47 (28 in genotype 1 and 19 in genotype 2a) agreed to additional sample for IL28B polymorphisms. The frequencies were 42 (89%) in CC type and 5 (11%) in CT type. There was no TT type. Their SVR rates were 91% (38/42) in CC type and 20% (1/5) in CT type. For genotype 1 (n=28), SVR rates were 88% (21/24) in CC type and 0% (0/4) in CT type. For genotype 2a (n=19), SVR rates were 89% (16/18) in CC type and 100% (1/1) in CT type. On multivariate analysis, IL28B-CC type (OR=38.001, 95% CI; 3.374-427.950, P=0.003) and EVR (OR=0.001, 95% CI; 2.435-351.427, P=0.001) were good predictors for SVR (Table 5).
Peginter feron and ribavirin combination is used as a standard regimen for the treatment of chronic hepatitis C (CHC) worldwide.14-16 The overall SVR rate in this retrospective study accounts for 81.2% in all genotypes, 75.5% in genotype 1, and 90.6% in genotype 2, all of which show higher SVR rate than other Korean studies.
According to studies by Kang et al17, SVR rates accounted for 69.5% in genotype 1, 89% in genotype 2/3, while studies by Kim et al18 indicated 60% of SVR rate in genotype 1, 86.1% in genotype 2/3. Also, studies by Kim et al19 showed higher SVR rates in genotype 1 (59.3%) and genotype 2/3 (90.5%), higher than numbers drawn out in studies by Lee et al20: 68.4% in genotype 1 and 94.1% in genotype 2/3. However, SVR rates have been reported as 42-51% in genotype 1 and 76-82% in genotype 2/3 in Western countries.14-16
The discrepancy among studies might be explained by some reasons. First, a high SVR rate may be explained by the high compliance percentage of patients available for follow-up observation after treatment type. In this study, the group using more than 75% of the total peginterferon dose showed a high SVR rate. It may be because compliance with treatment was so high that 51 among 79 patients available for SVR measurement were willing to use more than 75% of total peginterferon dose for their treatment. According to recent studies, differences in compliance with treatment showed significant differences in SVR rate between HCV genotype 1 and HCV genotype 2/3.19 Such results may help understand the importance of compliance with treatment to arrive at SVR. HCV genotype 1 is expected to require a higher rate of compliance than HCV genotype 2/3 because the former is understood to include a lower rate of compliance with treatment than the latter.14,15
Second, a higher rate of SVR may be explained by race. The genetic characteristics of patients may be prognostic factor that predicts antiviral response to peginterferon and ribavirin for chronic hepatitis C. Recently, single nucleotide polymorphisms (SNPs) near the IL28B gene have been reported as a predictor for responses to antiviral therapy in genotype 1 CHC patients.12,13 Although the precise mechanism responsible for the action of IL28B polymorphisms (rs12979860 CC type) is not fully understood, these genetic polymorphisms are located upstream of IL28B on chromosome 19 and are thought to have an effect on IL28B expression. IL28B, a cytokine that belongs to the IFN-family, is induced in response to viral infection, and can influence the downstream effects of IFN-α through signal transmission within the JAK-STAT pathway.10,11 Recently two studies in Korean have been reported. Lyoo et al21 showed that genotype rs12979860 CC and rs8099917 TT were more frequently observed in Korean patients compared to other ethnicities, and might be predicting factors for antiviral response. Sinn et al22 reported rs8099917 TT type accounted for 85% of Korean CHC patients, and it showed significantly low non-response rate than TG or GG type (2% vs. 22%, P=0.015) in genotype 1. Moreover, TT type showed a higher SVR rate than TG or GG type even though statistically non-significant (67% vs. 44%, P=0.191). Therefore we studied for IL28B genes in 47 patients. For rs12979860, the frequencies of polymorphisms were 89% in CC type, 11% in CT type, and 0% in CC type. Their overall SVR rate was 87% (39/47); 90.5% (38/42) in CC type and 20% (1/5) in CT type. The frequencies were 42 (89%) in CC type and 5 (11%) in CT type. There was no TT type. Their SVR rates were 91% (38/42) in CC type and 20% (1/5) in CT type. In accordance with Lyoo's report, we confirmed that IL28B CC type is frequent and associated with higher SVR in Korean CHC patients. This result can explain why Korean CHC patients had higher SVR rates than in Western countries.
Lastly, such a result may be explained by Koreans' comparatively lighter weight.23,24 Factors that have been found to affect SVR in previous studies are HCV genotype, copy of HCV RNA before treatment, result on liver biopsy, compliance with treatment, EVR, rapid virologic response (RVR), and race.25 However, effects on compliance with treatment that helps maintain appropriate drug injection have yet to be revealed. In this study, genotype and the total dose of peginterferon whereas HCV RNA copy before treatment, serum AST level, weight, EVR, presence/absence of cirrhosis did not indicate significant association. According to a study by Hadziyannis et al16 in 2004 on the treatment period and drug dose for HCV infection, the 48-week treatment group in HCV genotype 1 indicated 63% of SVR, an 11% gap for the 24-week treatment group's SVR. Also, as for genotype 2, 4 groups categorized by a 24-week treatment group, a 48-week treatment group, low dose ribavirin group (800 mg), standard dose group (1,000-1,200 mg) showed no significant differences in SVR.
A research into the solutions to the side-effects of treatment and premature halt in the course of a combination therapy using peginterferon and ribavirin is still insufficient. Side effects by peginterferon may differ among race; however, since Asians weigh lighter than westerners, the former group may suffer more side-effects from peginterferon alpha-2a whose dose is not adjusted in proportion to weight.
Previous studies reported that the discontinuation rates due to side effects were 6-13%.15,16,26 Discontinuation rate in current study, however, showed about 1.2%. This may be due to selection bias. On the other hand, there is a possibility that most people in the present study were tolerable to the treatment, which has resulted in a high SVR, 81.6% in genotype 1 and 96.7% in genotype 2. In present study, patients who showed abnormal hematologic findings accounted for 17.7-31.8% and one patient came to a stop treatment due to the reason. Asians may be thought to be more susceptible to hematologic adverse effects than westerners, but other Korean studies including present study indicated no severe side-effects such as sepsis and hemorrhage, and treatment proved effective after adjustment of drug dose. Clinicians should know treatment-related side effects and control them appropriately in order to increase the fidelity of treatment.
This present study has a few limitations. This study was conducted retrospectively with small number of patients in a single center. So this present study has a limitation by selection bias. In addition, we performed only one IL28B genotyping of rs12979860 and selected patients who agreed to human gene sequencing of IL28B. However, we found that nearly 90% of Korean CHC patients had homozygous genotypes for rs12979860 (CC), who showed high SVR. This result says why Korean CHC patients have a high SVR to the standard combination therapy.
We also identified through this research that the combination therapy with peginterferon plus ribavirin showed higher SVR than that of conventional interferon plus ribavirin treatment. Taken together, Korean CHC patients showed higher SVR rate and higher hematologic adverse effects when compared to those of Western people. The difference might be due to high prevalence of IL28B CC genotype in Korean. Large scaled prospective studies are needed to clarify these results. In addition, these results need to be considered in a treatment guideline for Korean CHC patients.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Grant No. 2011-0014391).
REFERENCES
2. Shin HR, Kim JY, Kim JI, Lee DH, Yoo KY, Lee DS, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: the role of acupuncture. Br J Cancer 2002;87:314-318. 12177801.




3. Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis 2000;20:1-16. 10895428.

4. Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777-782. 10706572.


5. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology 2000;31:1014-1018. 10733560.


6. Barrera JM, Bruguera M, Ercilla MG, Gil C, Celis R, Gil MP, et al. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology 1995;21:639-644. 7533121.


7. Kim YS, Pai CH, Chi HS, Kim DW, Min YI, Ahn YO. Prevalence of hepatitis C virus antibody among Korean adults. J Korean Med Sci 1992;7:333-336. 1284374.



8. Strader DB, Seeff LB. The natural history of chronic hepatitis C infection. Eur J Gastroenterol Hepatol 1996;8:324-328. 8781898.


9. Kim YS, Um SH, Ryu HS, Lee JB, Lee JW, Park DK, et al. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci 2003;18:833-841. 14676440.



10. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399-401. 19684573.



11. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105-1109. 19749757.



12. Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009;41:1100-1104. 19749758.



13. Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009;461:798-801. 19759533.



14. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965. 11583749.


15. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982. 12324553.


16. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355. 14996676.


17. Kang MJ, Jung EU, Park SW, Choi P, Kim JH, Park SJ, et al. Effects of pegylated interferon and ribavirin in Korean patients with chronic hepatitis C virus infection. Korean J Hepatol 2008;14:318-330. 18815455.


18. Kim YJ, Kim YI, Song YA, Jin NC, Lim SR, Ryang DY, et al. The effectiveness of combination therapy with peginterferon alpha-2a and ribavirin in chronic Hepatitis C. Chonnam Med J 2008;44:72-81.

19. Kim JI, Kim SH, Lee BS, Lee HY, Lee TH, Kang YW, et al. Efficacy of initial treatment with peginterferon alpha-2a versus peginterferon alpha-2b in combination with ribavirin in naive chronic hepatitis C patients living in Daejeon and Chungcheong Province in Korea: a comparative study. Korean J Hepatol 2008;14:493-502. 19119244.


20. Lee HJ, Eun JR, Choi JW, Kim KO, Moon HJ. Comparison of therapeutic results between combination therapy of peginterferon alpha-2a plus ribavirin and interferon alpha-2b plus ribavirin according to treatment duration in patients with chronic hepatitis C. Korean J Hepatol 2008;14:46-57. 18367857.


21. Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, Kim CW, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol 2011;52:363-366. 21907615.


22. Sinn DH, Kim YJ, Lee ST, Gwak GY, Choi MS, Lee JH, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol 2011;26:1374-1379. 21501223.


23. Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology 2002;36:1259-1265. 12395338.


24. Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med 2004;117:163-1638. 15276594.


Figure 1
Flow diagram of chronic hepatitis C patients. EVR, early virologic response; SVR, sustained virologic response.
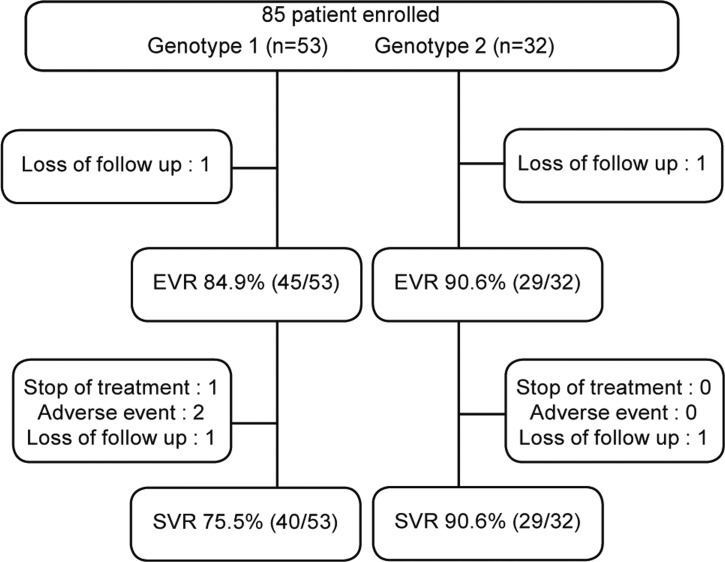
Table 1.
Baseline characteristics of the patients
| Factors | Genotype 1 (n=53) | Genotype 2 (n=32) | P-value* |
|---|---|---|---|
| Age (yr) | 42.1±10.6 | 47.6±9.5 | 0.017 |
| Gender (M/F) | 37/16 | 12/20 | 0.003 |
| BMI (kg/m2) | 23.7±3.0 | 23.5±2.4 | 0.784 |
| HCV RNA (×106 IU/mL) | 2.3±4.2 | 1.8±2.9 | 0.546 |
| DM, n (%) | 3 (5.70) | 2 (6.25) | 0.668 |
| HTN, n (%) | 6 (11.32) | 0 (0) | 0.079 |
| WBC (×103/µL) | 5.2±1.8 | 5.1±1.4 | 0.772 |
| Hemoglobin (g/dL) | 14.2±1.8 | 13.5±1.7 | 0.052 |
| Platelet (×103/µL) | 174±76 | 201±57 | 0.096 |
| AST (IU/L) | 66.7±55.9 | 54.6±41.4 | 0.295 |
| ALT (IU/L) | 93.7±80.6 | 69.9±59.9 | 0.157 |
| Total bilirubin (mg/dL) | 1.0±0.4 | 0.8±0.3 | 0.002 |
| Albumin (g/dL) | 4.3±0.3 | 4.2±0.4 | 0.201 |
| Liver cirrhosis, n (%) | 8 (15.09) | 3 (9.38) | 0.739 |
Table 2.
Factors associated with sustained virologic response (SVR) (univariate analysis)
| Factors | SVR (+) (n=69) | SVR (-) (n=16) | P-value* |
|---|---|---|---|
| Gender (M/F) | 39/30 | 10/6 | 0.667 |
| Age (yr±SD) | 44.0±10.5 | 44.7±10.5 | 0.818 |
| BMI (kg/m2±SD) | 23.6±2.7 | 23.4±3.1 | 0.741 |
| HCV genotype (1/2) | 40/29 | 13/3 | 0.034 |
| HCV RNA (×105 IU/mL) | 20.7±38.9 | 22.7±32.8 | 0.848 |
| Ribavirin dose (mg) | 1,123±105 | 1,109±98 | 0.465 |
| PEG-IFN dose (μg) | 163.2±26.3 | 162.7±22.8 | 0.016 |
| WBC (×103/μL) | 5.2±1.6 | 4.8±1.6 | 0.324 |
| Platelet (×103/μL) | 193.1±73.6 | 145.8±36.2 | 0.015 |
| ALT (IU/L) | 55.6±40.8 | 97.2±78.3 | 0.003 |
| Albumin (g/dL) | 4.3±0.3 | 4.1±0.3 | 0.101 |
| EVR | 64/67 | 11/16 | 0.001 |
Table 3.
Factors associated with sustained virologic response (SVR) (multivariate analysis)
| Factors | Odd ratios | 95% CI | P-value* |
|---|---|---|---|
| PEG-IFN total dose (≥75%) | 4.965 | 1.001-24.631 | 0.050 |
| Platelet (≥150×103/μL) | 1.642 | 0.286-9.419 | 0.578 |
| ALT (≥100 IU/L) | 3.300 | 0.671-16.215 | 0.142 |
| EVR (+) | 27.634 | 3.753-203.487 | 0.001 |
| HCV genotype 1 | 0.067 | 0.006-0.798 | 0.032 |
Table 4.
Adverse effects of combination therapy with peginterferon and ribavirin
Table 5.
Factors associated with SVR in chronic hepatitis C patients agreed with additional sample for IL28B polymorphisms
| Factors | SVR (+) (n=39) | SVR (-) (n=8) | P-value |
Multivariate |
||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value* | ||||
| Age (yr, mean) | 46.4 | 43.9 | 0.522 | |||
| Male No. (%) | 21 (54) | 2 (25) | 0.245 | |||
| HCV RNA (IU/mL, mean) | 1.6×106 | 3.3×106 | 0.116 | |||
| Platelet (×103/mm3) | 195 | 145 | 0.041 | |||
| >190×103/mm3, n (%) | 22 (56) | 1 (13) | 0.048 | 5.471 | 0.409-73.231 | 0.199 |
| ALT (IU/L, mean) | 72 | 113 | 0.299 | |||
| EVR | 39 (100) | 4 (50) | 0.001 | 30.250 | 2.435-351.427 | 0.001 |
| Genotype 1, n (%) | 21 (54) | 7 (88) | 0.081 | 0.167 | 0.019-1.486 | 0.827 |
| IL28B-CC type, n (%) | 38 (97) | 4 (50) | 0.002 | 38.000 | 3.374-427.950 | 0.003 |



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




